Acid Base Balance

Acid Base balance refers to the amount of acidity in the fluids. Something that is very acid will be caustic or “eats away certain things” it comes into contact with. Something that is very basic will also be caustic and “eat away certain things”. However, they are opposites. Acids “eat things” by giving up Hydrogen atoms (another way of saying protons). Bases “eat things” by taking away Hydrogen atoms. An example of an acid would be battery acid (sulfuric acid). An example of a base would be lye (sodium hydroxide). When the acids and bases are balanced the solution is considered neutral and produces essentially no effect on molecules that are contacted.
A scale has been devised for measuring the state of acid (or base) in solutions called the pH scale. This reflects the amount of the free Hydrogen atoms. It ranges from 1-14 with 1 being very acid, 14 being very basic (also called alkaline) and 7 is balanced or neutral. The acid base level (or pH) of tissues and fluids is very important since many functions in the body occur only at a certain level of acidity or alkalinity. Enzymes and chemical reactions are very dependent on the pH level of the surrounding fluids. A very small change in acid/base level can produce a profound change in these chemical reactions. I can remember in chemistry class adding a solution that was alkaline (basic) to another solution 1 drop at a time with no change until that key fraction of a drop was added. That fraction of a drop was enough to change the total mechanics of the chemical balance in the solution and the clear solution would instantly turn a bright color, cloud up and an entirely new chemical compound would settle out.
Knowing this you could see how crystals could settle into your joints as in gout and certain forms of arthritis, how stones might form in kidneys or gall bladder or possibly why certain things might be inclined to settle in vessels or in linings of tissues. Most tissues especially the blood, keep the acid base level within a very narrow range. When the tissue becomes too acid or too alkaline many problems appear. Acid base balance pays a direct role in the rate at which we burn our fuel for energy in the body. It plays a direct role in muscle contraction and in hormone production and release. It directly affects the nerves and nervous system, acidity causing sluggishness and drowsiness and alkalinity causing cramps, irritability and hyper excitability. Cancer development has been associated with localized over acidity of tissues. Proper acid/base balance is important to keep infection out of tissues including Candida or yeast.
Extremes of acidity and alkalinity occur in the digestive system in order to break down our food. The stomach may reach a pH of 1 which is very acid. If it cannot maintain proper acidity meat will not digest and problems result. Most stomach problems, by the way, result from not enough acid being produced rather than too much. Likewise, if the pancreas and other digestive organs fail to produce enough alkaline fluids to neutralize the stomach acid in the small intestine, enzymes that digest fat and carbohydrates will not work and foods do not digest.
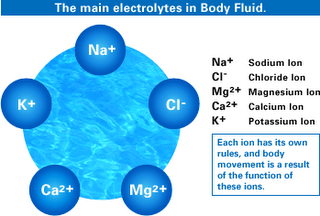
There are many mechanisms that the body uses in order to maintain the pH at the proper levels. Of these the kidneys and lungs play primary roles. The kidneys excrete extra hydrogen and retain extra sodium to reduce acidity. This requires phosphorous which may be taken from the bone if it is unavailable. If the blood is very acid the kidney excretes urea which gets rid of 4 hydrogens. If the blood is too alkaline, hydrogen is retained and sodium is excreted (the reverse). The lungs regulate acid base balance by varying the rate that carbon dioxide is breathed out. When carbon dioxide is in the blood it is in the form of carbonic acid which acidifies the blood. If you breathe rapidly the carbonic acid changes to carbon dioxide which is then breathed out of the lungs and the acid level of the blood goes down. If you breathe slowly the acid level rises. Digestive organs also regulate pH by the amount and balance of stomach acid and alkaline digestive juices that are secreted. The slight, short lived acid base changes that occur in the blood while digestion is occurring (known as acid and alkaline tides) reflects this. There also exist several specialized systems in the blood, lymph and fluids of the body that help maintain the proper acid to base balance. These are called buffer systems. A buffer system is one that changes an acid or base towards neutral. These include bicarbonate buffers, (many individuals used to take bicarbonate of soda for their acid stomach), protein buffers, fat buffers, and hormone buffers.
Lately there has been a trend saying that everyone must become alkaline in order to be healthy. In order to do this everyone must eat what are termed alkaline foods. These are foods that when burned leave an ash that is alkaline. If the ash is acid it is an acid food. Alkaline foods very generally are fruits and vegetables, acid foods are meats and grains. The truth is everyone must have a balanced pH that is normal for that part of the body in question. Symptoms develop when a person has areas of too much alkalinity just as symptoms develop when there is too much acidity. Saying that everyone must eat very high quantities of fruits and vegetables and little or no meat or it is impossible to be healthy simply doesn’t happen to be true. Some cultures in the arctic and high temperate zones eat no vegetables or only some during the very short summer and yet retain a very high quality of health.
The body is not as simplistic as saying meats and grains cause acidity and fruits and vegetables cause alkalinity. Meat that is not digested properly in the intestine for instance forms guanidine, an extraordinarily alkaline (and toxic) substance. One of the best buffers to control acid/base balance in the body is glutamine which has blood from meat as it’s best source. Phosphorus is necessary for kidneys to work properly in the correction of over acidity and the most common sources of phosphorous are grains. Organ meats can be very beneficial to key organs that help regulate acid/base balance.
It does seem to be true however, that with today’s modern diet of adulterated meats and foods, those who eat good fruits and vegetables fare better in general than those who don’t. In truth a lot of vegetables and fruits are good for the kidneys and lungs and digestive system and can produce very good effects on the system’s acid base balance. It really becomes a question of the positive effect being caused directly because of the acid or alkaline rating of each food as opposed to the positive effect being produced by the aid that the food gives the organs and systems that regulate the acid base balances. I suspect it is the latter.
The long and short of it is that acid/base balance is important and should be maintained at proper levels. This can be accomplished by giving the body the proper nutrients that are required. Should a person be short of vegetable based products, this can be supplemented. Should the person be short of kidney, lung, digestive or even blood and lymph support, this too may be supplemented depending on the specific needs of the individual. Determining which will work to restore each person’s acid base balance back to normal is possible through the use of Cause Point Correlative Testing.